汽化过氧化氢VHP灭菌技术的应用
汽化过氧化氢VHP灭菌技术的应用
汽化过氧化氢(Vaporized Hydrogen Peroxide,即缩写VHP)灭菌技术,是利用过氧化氢在常温下气体状态比液体状态更具杀孢子能力的优点,经生成游离的羟基,用于进攻细胞成分,包括脂类、蛋白质和DNA,达到完全灭菌要求的一种技术。常用于隔离室、隔离器等密闭空间的灭菌。汽化过氧化氢(VHP)灭菌干燥、作用快速、无毒无残留,物质相容性较好,包括很多金属和塑料,适用于房间、生物安全柜、传递窗、动物笼交换站、隔离器和医疗器械等表面的灭菌消毒。生物净化时间短,根据待处理产品的物理特性,生物灭菌时间30~90min,对更广范围的微生物有效,生物灭菌循环中不产生有毒残留物,对于其他物品影响不大(装置、电器、洁净室墙板等)灭菌所需时间短,容易验证。
一、VHP过氧化氢消毒技术的背景
制药厂传递窗的灭菌方法主要包括消毒液擦拭法、紫外灯照射法、甲醛熏蒸消毒、二氧化氯气体消毒等,这些方法存在可重复性差、难于验证、破坏性大、需消耗大量的人力,以及会危害作业人员的身体健康等特点。在当今制药工业中,过氧化氢已逐渐取代传统灭菌方式,成为表面或空间消毒及灭菌的常用方法,过氧化氢由水和氧气组成,两者均很安全,在杀菌有效性、人员安全性、材料兼容性以及环境有好性方面具有明显优势。
对于人类来说,暴露在一定浓度水平的消毒气体中是有害健康的,这个浓度水平因不同的气体而不同。根据美国职业安全与健康管理局( OSHA)公布的数据,现将甲醛熏蒸、二氧化氯气体、汽化过氧化氢三种消毒技术安全性方面的指标比较见表。
| 三种化学气体消毒技术的安全指标比较 | |||
|---|---|---|---|
| 主要项目 | 三种气体消毒技术安全指标(mg/L) | ||
| 过氧化氢 | 二氧化氯 | 甲醛 | |
| 直接接触 | 皮肤眼睛刺激 | 严重刺激性 | 人体致癌 |
| PEL | 1.0 | 0.1 | 0.75 |
| STEL | - | 0.3 | 2 |
| IDLH | 75 | 5.0 | 10 |
| 灭菌浓度 | 150-700 | 350-1500 | 8000-10000 |
| 注:PEL,8h允许暴露限值。STEL,15min短时暴露限值。IDLH,生命与健康造成急性危害的限值。 | |||
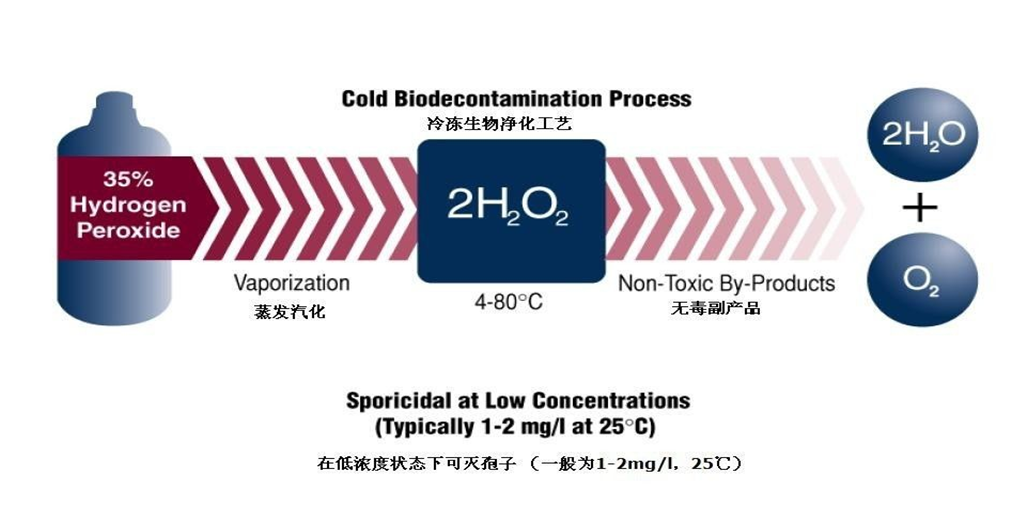
过氧化氢早在100多年前就证明具有良好的杀菌作用,可杀灭各种细菌、真菌、病毒及细菌芽孢,其3.0%水溶液可用于人体皮肤粘膜的局部消毒,6.0%以上浓度可用于耐腐蚀物品的高水平消毒。其杀菌作用原理主要通过氧化作用,以及分解后产生的自由基团,破坏微生物的蛋白质、氨基酸、酶和DNA,破坏微生物的通透性屏障,最终导致微生物死亡。
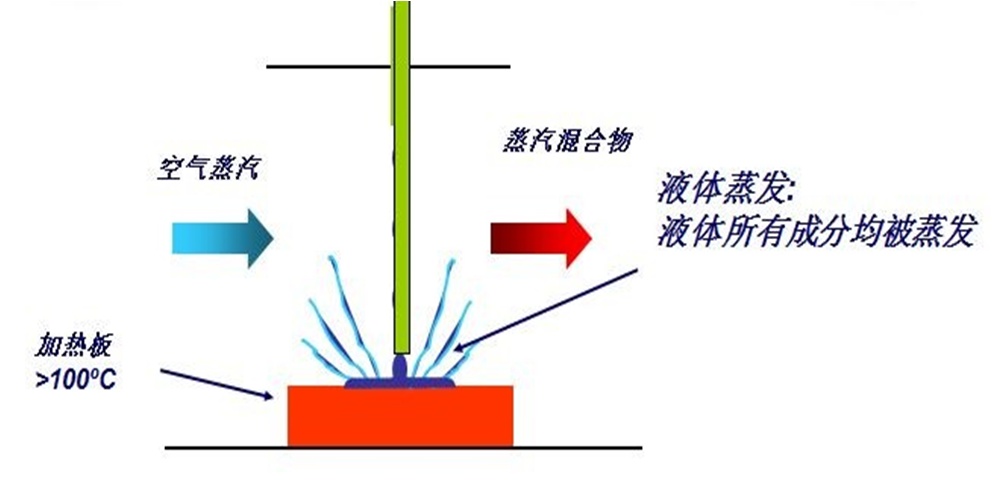
液体变成气体,需要加温汽化,一般水的汽化温度为100℃,纯的过氧化氢汽化温度为150.2℃,而该技术使用30% - 35%过氧化氢溶液的汽化温度大约为108℃。汽化过氧化氢灭菌技术必须使用一种加热器,或称过氧化氢蒸汽发生器,通过所谓的“闪蒸”将液态过氧化氢转化为VHP,此过程可在常温环境下有效进行。
空气主要由氮气、氧气、水气和少量惰性气体组成。空气中含多少水气肉眼无法直观看到,但是可以通过仪器测定,空气中含水气量的多少,一般用相对湿度( RH)表示。在温度降低时,空气中的水气会形成极小的雾粒,水气越大温度越低,达到“凝露”点时,就会形成露水,沉降下来。
同样的原理,较高温度的VHP被均匀的引入密闭空间,一般维持的VHP气体浓度为150 - 700 mg/L,遇到空间内相对较低温度时,就会形成1微米纳米雾(又称干雾),蒸汽和纳米雾都具有极其容易穿透各种物体的特性,使污染的表面完全暴露于VHP中,附着寄居的微生物表面,在常温下接触到VHP,还会进一步形成微冷凝,理论上冷凝后VHP浓度会迅速升高(水冷凝后浓度100%,VHP冷凝后过氧化氢浓度可达70%以上),并快速杀灭各种微生物。
研究发现当目标环境在VHP循环过程中的浓度达到峰值时,会在所有暴露物体的表面形成一层看不到的沉积物,显微镜下是一个约2μm的冷凝薄膜,产生更为有效的杀灭作用。一旦过氧化氢分子接触到物体表面,即刻产生氧化作用形成自由基攻击微生物,达到高水平的消毒灭菌,并且对细菌、孢子、真菌、霉菌和病毒都广谱有效。
整个过程通过计算机和彩色触摸屏在密闭空间外进行控制,并实时反馈循环进程,被VHP消毒的空间或设备需要密封起来,通过电化学原理的手持式VHP传感器监测没有泄露发生,以及环境是否在循环后恢复至可以进入的安全水平。
二、VHP消毒技术优势
1、安全
使用蒸汽灭菌,在整个灭菌过程中限制人员进入,若灭菌失败可安全复原。
2、可靠
通过汽化过氧化氢灭菌处理,可以使得密封屏障内空气与表面杀菌对数达到4~6,整个灭菌过程简单可靠。
3、智能化
能够实现与建筑设施一体化,与BMS实现控制一体化,整个消毒过程可更换操作人员,降低对操作人员依赖性。
4、标准化
将过氧化氢技术与空调系统结合,能够有效避免传统灭菌难以标准化、验证困难等问题。
目前,制药厂房中常见的过氧化氢消毒方式主要包括汽化过氧化氢、气源式雾化过氧化氢和电源式雾化过氧化氢灭菌。
| 3种过氧化氢消毒方式比较 | ||||||||||||
| 观察项目 | 浓度(g/L) | 消耗量(ml/m³) | 载气手段 | 均流方式 | 消毒时间(h) | 温湿度 | 杀灭对数值 | 腐蚀性 | 操作人员数 | 去残留时间(min) | 空气中浓度(mg/m³) | 辅助设施 |
| 汽化 | 300~600 | >20 | 内外风机 | 外部风机 | 5~8 | 30℃,30% | >6.0 | 明显 | 3~5 | >30 | ≥250 | 均流单元 |
| 气源雾化 | 复配过氧化氢 | >20 | 压缩空气 | 压缩空气 | >4 | 常温常湿 | >4.0 | 有腐蚀性 | 3~5 | 风扇 | ||
| 电源雾化 | 80g/L与5%乙醇混合 | >8 | 自带风机 | 无需风机 | 1~3 | 25℃,50%~70% | >6.0 | 腐蚀性较低 | 1~2 | >30 | 无需 | |
三、VHP消毒工作原理
通过高温闪蒸将过氧化氢液体汽化成过氧化氢气体,经高速气流喷射到灭菌空间中,当高温饱和过氧化氢蒸汽接触到较冷的被消毒物品表面时,会形成不可见的微冷凝,通过释放的强氧化自由基(羟基)来攻击病原微生物(破坏细胞膜、脂类、蛋白质和DNA),自由基可对微生物达到log6的快速杀灭效果。灭菌完成后,汽化过氧化氢灭菌设备会自动将环境中的过氧化氢分子分解为水蒸气和氧气,当空间内过氧化氢浓度降至1ppm以下时人员方可重新进入洁净区。
 |
 |
汽化过氧化氢灭菌过程分为以下四个操作阶段:
采用设施的通风系统将蒸汽从目标区域移除,或者兼用通风单元快速催化分解蒸汽为水和氧,在设施的通风系统没有合适的阀门或者不易控制时,采用独立的通风系统将很有意义。
四、VHP空间灭菌在生物安全消毒领域的应用
VHP技术已经应用了20年之久,全球制药行业85%以上无菌隔离器使用VHP技术作为生物去污染的选择。迄今,VHP已经被广泛应用于隔离器、空气过滤器、无菌灌装线、高等级生物安全实验室、传染病病房、隔离担架、救护车等不同领域消毒处理。
采用对灭菌房间的直接喷射(喷头一般置于房间的中心位置,直接喷射VHP进入环境,即不需要通过过滤器处理),过氧化氢蒸汽直接喷射进工作区,而不是通过供气和排气的高效过滤,加上去除了对环境条件的限制,循环时间在1 h内得以完成,在某些环境下也明显的改善了工作流程。
进行大面积空间消毒时,消除对任何环境条件的限制是必需的,国外在生物恐怖污染消除、突发烈性传染病消毒、生物实验室污染消毒等领域。国内在生物安全三级实验室、动物实验室、大型集装箱组、食品无菌加工的污染消除。
国内在生物安全三级实验室、动物实验室、大型集装箱组、食品无菌加工的污染消除方面有很多成功的报告。
五、验证
验证是中外制药企业GMP管理的重要组成内容。验证的定义最早见于美国食品药品监督管理局FDA公布的《药品工艺检查验收标准》(1978年6月):“一个已验证的工艺系指已能证实按预计或所声称的那样运行的工艺。验证的证据是通过尽可能收集和评估工艺开发阶段的数据,以及以后生产阶段的数据获得的。验证必须包括工艺确认(材料、设备、系统、建筑及人员的确认),以及重复性生产的批或运行的整个工艺的控制”。
我国新颁布的《药品生产质量管理规范(2010年修订)》在第十四章第三百一十二条将验证定义为“证明任何操作规程(或方法)、生产工艺或系统能够达到预期结果的一系列活动”。GMP中验证概念的引入标志着质量管理从“质量检验”提升至“质量保证”,被称为是GMP发展史的里程碑。
无菌药品洁净区域的化学气体熏蒸灭菌,要求其可安全地使用于不锈钢、钢、塑料、玻璃、环氧地面、墙壁等各种表面,并对包括孢子在内的微生物进行快速、有效的灭菌控制,能通过对生物指示剂挑战性试验。
消毒灭菌方法的验证是极其重要的,只有通过验证,才能肯定消毒灭菌方式的有效性和安全性。无菌药品的要求采用了最新的世界卫生组织和欧盟 A、B、C、D 分类,对无菌药品生产的洁净度、级别提出了具体要求,要求达到动态A级的标准,增加了在线监测,特别是对悬浮粒子的静态、动态监测,对浮游菌、沉降菌、生产环境中的微生物和表面微生物的监测都作了详细的规定。
| 洁净区微生物监测的动态标准⑴如下: | ||||
| 洁净度级别 | 浮游菌cfu/m³ | 沉降菌(Φ90mm)cfu/4小时⑵ | 表面微生物 | |
| 接触(Φ55mm)cfu/碟 | 5指手套cfu/手套 | |||
| A级 | <1 | <1 | <1 | <1 |
| B级 | 10 | 5 | 5 | 5 |
| C级 | 100 | 50 | 25 | - |
| D级 | 200 | 100 | 50 | - |
|
注: ⑴表中各数值均为平均值。 ⑵单个沉降碟的暴露时间可以少于4小时,同一位置可使用多个沉降碟连续进行监测并累积技术。 |
||||
目前汽化过氧化氢灭菌法已成为各国药典、药品生产质量管理规范(GMP)、消毒灭菌技术规范所推荐的方法,灭菌工艺已经非常成熟,重复性好,有专门的商业化的非常标准的化学指示剂和生物指示剂用来检测灭菌过程中过氧化氢气体分布的均匀性和最终的灭菌效果。
上海杰昊生物技术有限公司研发生产的VHP汽化过氧化氢发生器采用最新的第三代干法闪蒸技术,不仅闪蒸温度控制稳定,汽化完全,产生的过氧化氢气体粒径小,而且在灭菌的不同阶段可以设置不同的注入速率,在保证灭菌效果前提下,提升物料兼容性;相对于闭环终端喷放过氧化氢气体,JIEHAO VHP汽化过氧化氢发生器采用开环开放式气体循环方式,气体分布系统的配合使用更使过氧化氢气体分布均匀,有效扩展消毒灭菌体积,降解速度也得到了较大的提升。








